A portable power supply has become the lifeline of the modern technological world, especially lithium-ion batteries.
Imagine a world where all cars run on induction motors and not internal combustion engines. Induction motors are far superior to IC engines in almost all engineering aspects, as well as being more robust and cheaper.
Another major disadvantage of IC engines is that they produce usable torque only in a narrow band of engine RPMs. Considering all these factors, induction motors are definitely the right choice for an automobile. However, the power supply for an induction motor is the real impediment in achieving a major induction motor revolution in the automobile industry.
Let's find out how Tesla solved this issue with the help of lithium-ion cells and why lithium-ion cells are going to become even better in the future.
Let's take a Tesla cell out of the battery pack and break it down. You can see different layers of chemical compounds inside it. Tesla's lithium-ion battery works on an interesting concept involving metals called electro-chemical potentials.
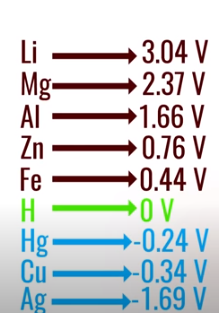
Electro-chemical potential is the tendency of a metal to lose electrons. In fact, the first cell, developed by Alessandro Volta more than 200 years ago, was based on the concept of electro-chemical potential.
According to these values, lithium has the highest tendency to lose electrons and fluorine has the least tendency to lose electrons.
Volta took two metals with different electro-chemical potentials, in this case, zinc and silver, and created an outward current of electricity.
The Sony Company produced the first commercial model of lithium-ion batteries in 1991. It was again based on the same concept of electro-chemical potential.
Lithium, which has the highest tendency to lose electrons, was used in lithium-ion cells. Lithium has only one electron in its outer shell and it always wants to lose this electron.
For this reason, pure lithium is a highly reactive metal. It also reacts with water and air. The trick to the operation of a lithium-ion battery is the fact that lithium in its purest form is a reactive metal.
But when lithium is part of a metal oxide, it is quite stable. Let's say that somehow we have. This separated the lithium atom from the metal oxide. This lithium atom is highly unstable and will immediately form a lithium-ion and an electron. However, lithium as part of the metal oxide is much more stable than in this state. If you can provide two separate paths for electron and lithium-ion flow between the lithium and the metal oxide, the lithium atom will automatically reach the metal oxide part.
During this process, we have produced electricity by electron flow through a path. From these discussions, it is clear that we can produce electricity from this lithium metal oxide, if we first separate the lithium atoms from the lithium metal oxide, and secondly, the lost from such lithium atoms through an external circuit. Guide the electrons.
Let's discuss how lithium-ion cells achieve these two purposes. A practical lithium-ion cell also uses an electrolyte and graphite. Graphite has a layered structure. These layers are loosely bound so that the dissociated lithium-ions can be easily stored there.
The electrolyte between the graphite and the metal oxide acts as a guard that only lets the lithium-ions in. Now let's see what happens when you connect a power source to this arrangement.
The positive side of the power source will obviously attract and remove electrons from the lithium atoms of the metal oxide.
These electrons flow through the external circuit because they cannot flow through the electrolyte and reach the graphite layer. Meanwhile, the positively charged lithium-ions will be attracted to the negative terminal and flow through the electrolyte.
Lithium-ions also reach the site of the graphite layer and get trapped there. Once all the lithium atoms have reached the graphite sheet, the cell is fully charged. Thus we have achieved the first objective which is to separate lithium-ions and electrons from metal oxides.
As we discussed, it is an unstable state, as if situated on top of a hill. As soon as the power source is removed, and a load is connected, the lithium-ions want to return to their steady state as part of the metal oxide.
Because of this tendency, lithium-ions move through the electrolyte and electrons move through the load, as if sliding down a hill. Thus we get electric current through the load. Please note that graphite does not play a role in the chemical reaction of lithium ion cells.
Graphite is the storage medium for lithium-ion. If an abnormal condition causes the internal temperature of the cell to rise, the liquid electrolyte will dry up and a short circuit will occur between the anode and cathode, and this may result in a fire or explosion.
To avoid such a situation, an insulating layer, called a separator, is placed between the electrodes. The separator is permeable to lithium-ions due to its fine porosity. In a practical cell, graphite and metal oxides are coated on copper and aluminium foil.
Files act as current collectors here and positive and negative tabs can be easily removed from current collectors. An organic salt of lithium acts as the electrolyte and is coated on the separator sheet.
These three sheets are wound on cylinders around a central steel core, thus making the cell more compact. A standard Tesla cell has a voltage of between three and 4.2 volts. Several such Tesla cells are connected in series and in parallel fashion to form a module. 16 such modules are connected in series to form a battery pack in a Tesla car.
Lithium-ion cells generate a lot of heat during operation and high temperatures will degrade the performance of the cells. A battery management system is used to manage the temperature, state of charge, voltage protection and cell health monitoring of such a large number of cells. Glycol based cooling technology has been used in Tesla battery pack.
The BMS adjusts the glycol flow rate to maintain optimum battery temperature. Voltage protection is another important function of BMS. For example, during charging in these three cells, the cell with higher capacity will be charged more than the rest.
To get around this problem, BMS uses something called cell balancing. In cell equilibrium, all cells are allowed to charge and discharge equally, thus protecting them from over and under voltages. This is where Tesla has left Nissan battery technology behind.
There is a major problem with cooling the battery due to the large size of the Nissan Leaf's cells and the absence of an active cooling method. The smaller multiple cell design has another advantage.
During conditions of high power demand, the discharge strain will be divided equally across each cell. If we had used one giant cell instead of many smaller cells, it would have come under a lot of pressure, and eventually it would have died prematurely.
By using several small cylindrical cells, the manufacturing technology of which is already well established, Tesla clearly made a winning decision.
There is a magical event that occurs within lithium-ion cells during their first charge that saves lithium-ion cells from sudden death.
Let's talk about what it is. Electrons are a major problem in the graphite layer. The electrolyte will degrade if electrons come into contact with it. However, due to the accidental discovery, the solid electrolyte interface the electrons never come into contact with the electrolyte.
When you first charge the cell, as mentioned above, lithium-ions move through the electrolyte. Here, in this journey, the solvent molecules in the electrolyte cover the lithium-ion. When they reach the graphite, the lithium-ions, along with the solvent molecules, react with the graphite and form a layer there called the SEI layer.
Creating this SEI layer is a boon in disguise. This prevents any direct contact between electrons and the electrolyte, thus protecting the electrolyte from degradation.
In this overall process of SEI layer formation, it will consume 5% of lithium. The remaining 95% lithium contributes to the main function of the battery. Even though the SEI layer was an accidental discovery, with more than two decades of research and development, scientists have optimized the thickness and chemistry of the SEI layer for maximum cell performance.
It is surprising to find that almost two decades ago most of the electronic gadgets we used did not use lithium-ion batteries. With its amazing pace of growth, the lithium-ion battery market is expected to become a $90 billion annual industry within a few years. The number of charge-discharge cycles currently achieved for a lithium-ion battery is about 3,000. Great minds around the world are doing their best to increase this to 10,000 cycles. That is, you will not have to worry about changing your car battery for 25 years.
Millions of dollars have already been invested in research to replace the storage medium graphite with silicon. If it is successful, the energy density of the lithium-ion cell would increase by more than five times.






Comments
Post a Comment
If you have any Doubts plz let me know